November 22, 2025
This document outlines the step-by-step instructions for requesting access to the following Digital Research Data Repositories:
• Complete PDSR Curated Data Set
• COVID-19 Data Mart & Detailed Data Files in Enclave
• COVID-19 Vaccine Registry in Enclave
• COVID-19 Vaccine Self-Reported Symptoms in Enclave
• ECG Data Files in Enclave
• Bring Your Own Data (BYOD) in Enclave
• Industry Sponsored Data Marts
• COVID-19 Summary Table in SFA (Shared File Area)
• MGB COVID Registry in SFA
• User Work Datamart
Introduction
Researchers use the ServiceNow Researcher Access Request Form to request access to several Digital Research data repositories.
New Project Requirements Before Submitting (Excludes Bring Your Own Data BYOD Projects)
• The Project Lead must be an RPDR Faculty Sponsor (i.e. RPDR Workgroup Leader). Please see RPDR Confidentiality for more information or RPDR Registration to register as a Faculty Sponsor.
• All Project Staff must be a member of the Project Lead's Workgroup in RPDR.
The form is divided into sections for easy navigation:
• User Requesting Access: Questions about the requestor
• Workgroup Project Details: Select or create the workgroup project and select data to access
• Access Details: Questions about data repository access
• Affirm: Attest to policies and/or sign DUA (if applicable)
Once submitted, the access request is reviewed for accuracy. The request is then sent for approval by one or more of the following individuals: project lead, PI, data owner(s). After all required approvals are received, the access provisioning process starts and once completed, the requestor receives instructions on how to access the data repository. Please note: The requestor is notified via ServiceNow automated emails thru every step of this process.
Provided approvals are received in a timely manner, please allow 1-7 business days for access to be provisioned.
Requirements
- Mass General Brigham (MGB) credentials (username and password) are required for submitting the access request to a Digital Research Data Repository.
- Ensure you are on a MGB network or have established a MGB VPN connection. | KB0023967
- You have reviewed the respective data repository dashboard and data dictionary listed on the Digital Research Main Dashboard in Collibra.
- If your research project or study includes an approved active IRB protocol, you must be a staff member of the IRB protocol before submitting access.
- For access to a Patient Cohort User Work Datamart, the datamart has been provisioned and you know the datamart name. (Example: PROJXXXX_Userwork)
Step 1: Access the ServiceNow Researcher Request Form
Click this ServiceNow Researcher Access Request Form link to access the form. You may be asked to authenticate so be ready to provide your MGB credentials.
Step 2: Review User Requesting Access Section
The User Requesting Access section should be pre-filled with your name and your manager’s information. Select your employment status and your primary affiliation. Please review for accuracy and ensure all required fields are populated.
Step 3: Select an existing Project or create a new Project
For data repositories in the Enclave, your research project defines your Enclave workspace. If your project was created previously by a project team member, you can search and select it in the Project Name field. Otherwise, you will need to create a new project. For User Work Datamart access, search and select using your Project ID (Example: PROJXXXX).
To search for your existing project:
1. Within the Project Details section of the form, click on the triangle next to the Project Name input box. You can also search for your Project by typing in a Project Detail (Project Name, Project ID, Project Lead, IRB Protocol ID, PI, or Project Status).
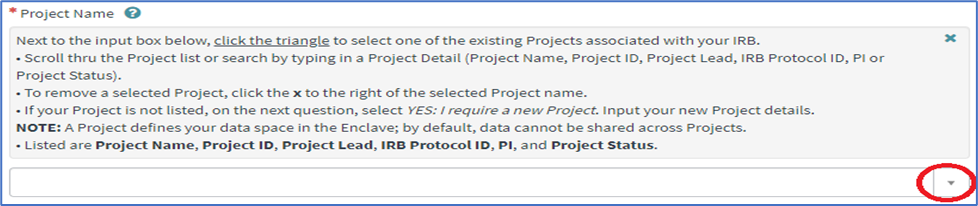
2. Scroll thru the dropdown to select the appropriate project. Once selected, your request form will be populated with the project details. If you do not see your project listed, you will need to create a new project.
Please note: To select a project with an IRB protocol, you must be a staff member of the protocol before submitting access.
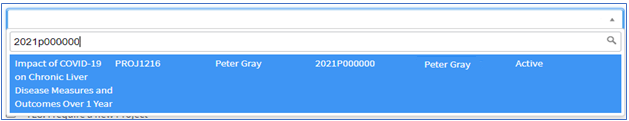
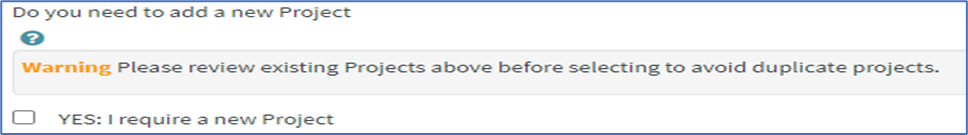
To create a new project:
Available to MGB employees only. Do not select for User Work Datamart access.
1. Click Yes: I require a new Project checkbox to create a new project.
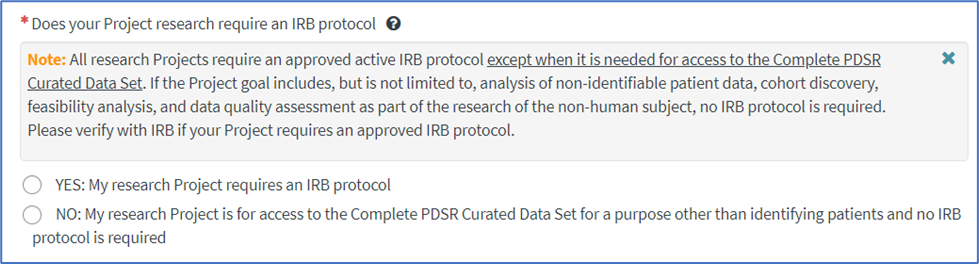
2. Select the appropriate response to Does your Project research require an IRB protocol. IMPORTANT: If you are accessing the Complete PDSR Curated Data Set for the purposes other than identifying patients and you do not have an IRB protocol, you must select NO. Otherwise you must select YES, have an active IRB protocol, and the protocol must contain verbiage for use of the repository data.
3. Enter your Project Name and Project Description.
4. Search for/select the Project Lead Name by entering the First and Last name or preface with an asterisk (*). Examples: Joe Public, *Public. Please wait a second or two and select the listed name.
5. If your project requires an IRB Protocol, enter the approved active IRB Protocol Number. You must be a staff member of the protocol before submitting access.
Please note: New projects without an IRB protocol will have a Project End Date set to one year from the date of project creation.
Step 4: Select data to access
After selecting or creating your project, choose the appropriate response to Select which data you need for this project.

• Select COVID-19 Data for access to either the COVID-19 Data Mart & Detailed Data Files, COVID-19 Vaccine Registry, or COVID-19 Vaccine Self-Reported Symptoms data marts.
• Select User Work Datamart For Your Project for access to your projects patient cohort datamart.
• For access to the Complete PDSR Curated Data Set, confirm how you plan to use the data:
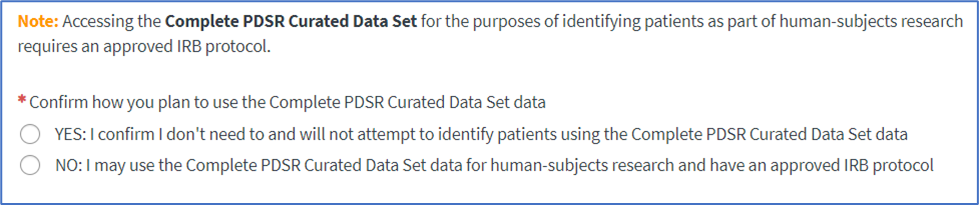
• A Yes indicates you will not attempt to identify patient data and an IRB protocol is not required.
• A No indicates an approved project IRB protocol is required and you must confirm your IRB protocol contains specific language to use the PDSR Curated Data Set.
If you are unsure, please reach out to the IRB for clarification before submitting your access.

Step 6: Review, Affirm, and Submit
1. Review the entire form and ensure all required fields denoted with an asterisk(*) are populated.
2. Access to the Complete PDSR Curated Data Set requires you sign the Date Use Agreement (DUA) by providing your name.
3. Accessing data with an approved project IRB protocol requires you attest to compliance with the allowances of your Mass General Brigham sponsored IRB human studies protocol.
4. Click Submit on the right side of the page. If Errors appear, resolve them and continue to click Submit until all errors are resolved.
Please allow 1-10 business days to provision your access. For additional assistance please contact MGBAnalyticsEnclaveSupport@mgb.org.
Additional Resources
Digital Research Main Dashboard in Collibra
